How To Teach The Table Of Standard Reduction Potentials
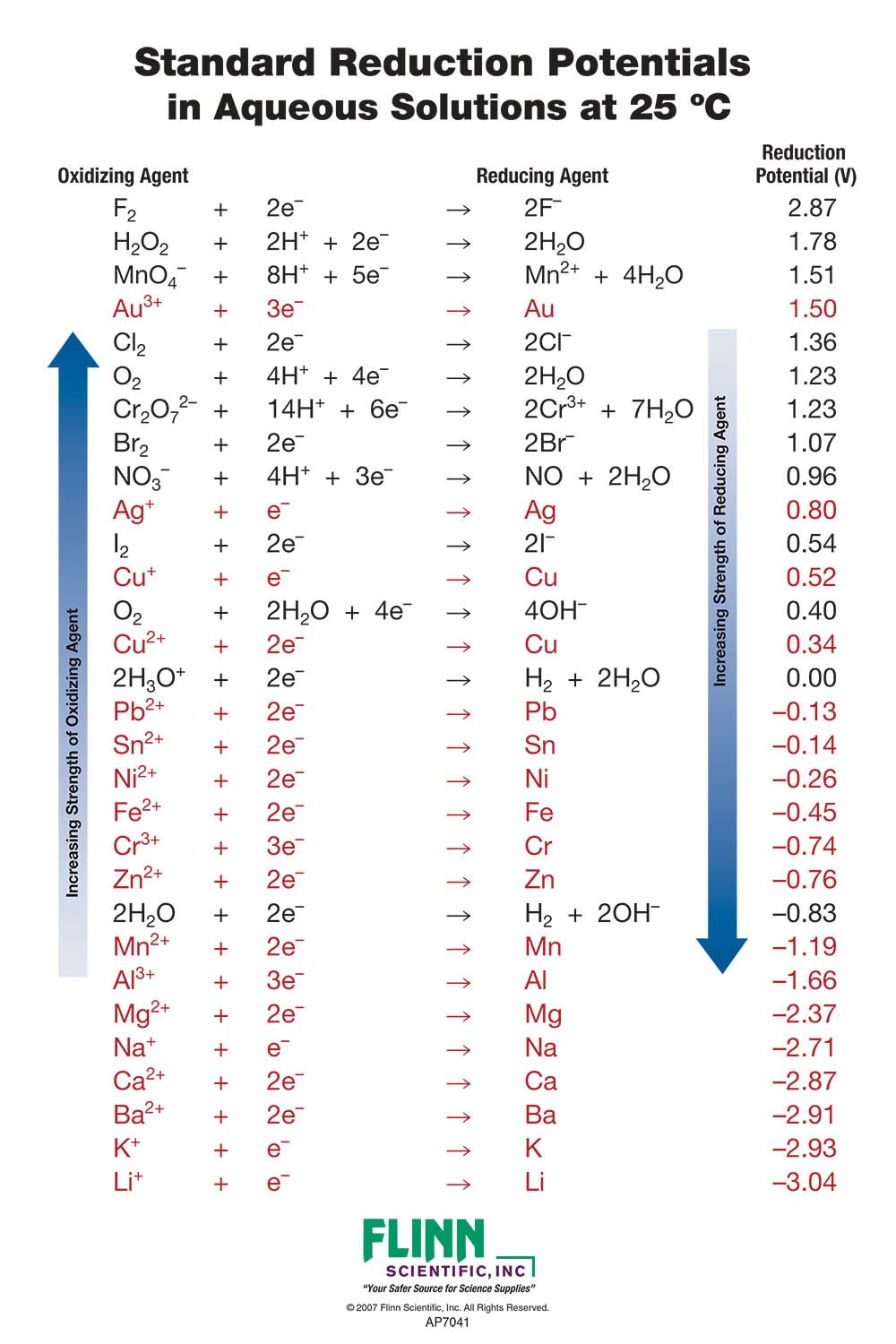
Standard Reduction Potential Charts For Chemistry The table is ordered such that the stronger (more reactive) reductants are at the top and the stronger oxidants are at the bottom. standard cathode (reduction) half reaction standard reduction potential e° (volts). Using table 17.3.1, the reactions involved in the galvanic cell, both written as reductions, are. au3 (aq) 3e − au(s) e ∘ au3 au = 1.498v. ni2 (aq) 2e − ni(s) e ∘ ni2 ni = − 0.257v. galvanic cells have positive cell potentials, and all the reduction reactions are reversible. the reaction at the anode will be the half.

Table Of Standard Reduction Potentials Youtube Table of standard reduction potentials potentials are given in the table in . volts. relative to the . standard hydrogen electrode and are for the following. Standard reduction potentials made easy. standard reduction potentials are very useful in chemistry. they are also known as standard cell potentials, or standard electrode potentials. they are measured in volts, and they tell you how likely an element or ion is to be reduced by gaining electrons. we explain the concepts clearly, and give you a. The she is rather dangerous and rarely used in the laboratory. its main significance is that it established the zero for standard reduction potentials. once determined, standard reduction potentials can be used to determine the standard cell potential, e cell °, e cell °, for any cell. for example, for the cell shown in figure 17.4,. How are standard reduction potentials applied. standard reduction potentials are used to determine the standard cell potential. the standard reduction cell potential and the standard oxidation cell potential can be combined to determine the overall cell potentials of a galvanic cell. the equations that relate these three potentials are shown below:.
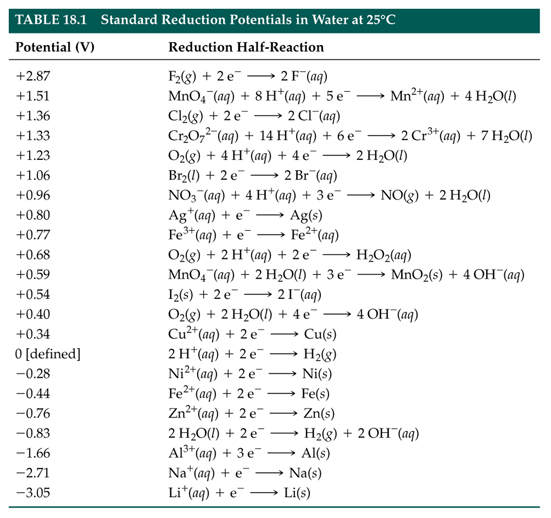
Lesson 5 Standard Reduction Potentials Latest Copy Of Grade 12 U The she is rather dangerous and rarely used in the laboratory. its main significance is that it established the zero for standard reduction potentials. once determined, standard reduction potentials can be used to determine the standard cell potential, e cell °, e cell °, for any cell. for example, for the cell shown in figure 17.4,. How are standard reduction potentials applied. standard reduction potentials are used to determine the standard cell potential. the standard reduction cell potential and the standard oxidation cell potential can be combined to determine the overall cell potentials of a galvanic cell. the equations that relate these three potentials are shown below:. How to use a table of standard reduction potentials to calculate standard cell potential. identifying trends in oxidizing and reducing agent strength. watch. The reduction half reaction chosen as the reference is. 2h (aq,1m) 2e− ⇌ h2(g,1 atm) e∘ = 0 v 2 h (a q, 1 m) 2 e − ⇌ h 2 (g, 1 atm) e ∘ = 0 v. e ° is the standard reduction potential. the superscript “°” on the e denotes standard conditions (1 bar or 1 atm for gases, 1 m for solutes). the voltage is defined as zero for all.

Standard Potential Pveducation How to use a table of standard reduction potentials to calculate standard cell potential. identifying trends in oxidizing and reducing agent strength. watch. The reduction half reaction chosen as the reference is. 2h (aq,1m) 2e− ⇌ h2(g,1 atm) e∘ = 0 v 2 h (a q, 1 m) 2 e − ⇌ h 2 (g, 1 atm) e ∘ = 0 v. e ° is the standard reduction potential. the superscript “°” on the e denotes standard conditions (1 bar or 1 atm for gases, 1 m for solutes). the voltage is defined as zero for all.
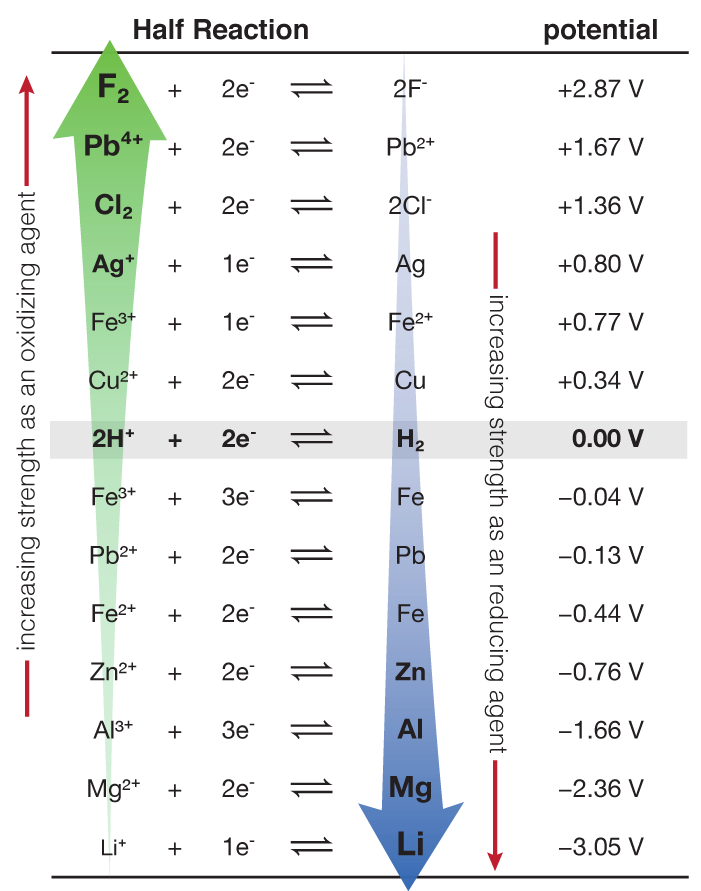
Standard Reduction Potentials Chart

Comments are closed.