Determination Of Hardness Of Water

Determination Of Hardness Of Water By Edta Method Youtube Learn how to use edta reagent to measure the total hardness of water by forming complexes with calcium and magnesium ions. compare different procedures based on buffer solution, indicator, and titrant. Hardness* 2340 a. introduction2340 hardness*2340 a.introduction1. definitionoriginally, water hard. ess was understood to be a measure of the capacity of water to precipit. te soap. soap is precipitated chiefly by the calcium and magnesium ions present. other poly valent cations also may precipitate soap, but they often are in complex forms.

How To Determine Hardness Of Water By Edta Method Procedure And Problems On Hardnessо The hardness of water is defined in terms of its content of calcium and magnesium ions. since an analysis does not distinguish between ca2 and mg2 , and since most hardness is caused by carbonate deposits in the earth, hardness is usually reported as total parts per million calcium carbonate by weight. a water supply with a hardness of 100. Learn how to measure the total hardness of water by titrating it with edta and eriochrome black t indicator. the experiment involves buffer solution, inhibitor, naoh, standard solutions and apparatus. There are some reagents needed for the determination of hardness of water which is given below: 1. standard edta titrant (0.01 m) 2. ebt (eriochrome black t) indicator and. 3. ammonia buffer solution. procedure for the determination of hardness of water. first, take a 50 ml water sample into a conical flask. It is water insoluble and is the main component of the scale that clogs pipes. concentrations of mg2 and ca2 are much higher than any other ions responsible for hardness, and total water hardness is defined as the sum of the calcium and magnesium concentrations. total water hardness is usually expressed as the milligrams of caco3 equivalent.
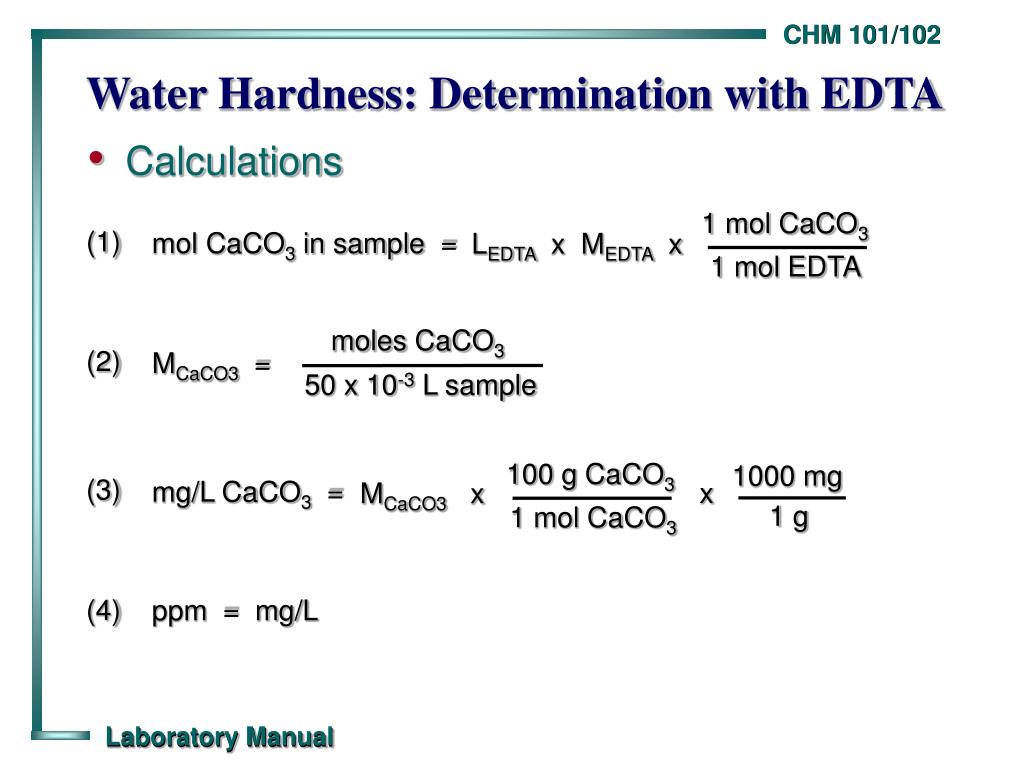
Ppt Water Hardness Determination With Edta Powerpoint Presentation Free Download I There are some reagents needed for the determination of hardness of water which is given below: 1. standard edta titrant (0.01 m) 2. ebt (eriochrome black t) indicator and. 3. ammonia buffer solution. procedure for the determination of hardness of water. first, take a 50 ml water sample into a conical flask. It is water insoluble and is the main component of the scale that clogs pipes. concentrations of mg2 and ca2 are much higher than any other ions responsible for hardness, and total water hardness is defined as the sum of the calcium and magnesium concentrations. total water hardness is usually expressed as the milligrams of caco3 equivalent. Learn how to measure total water hardness by complexometric titration with edta and eriochrome black t indicator. find reaction, procedure, result calculation and sources of errors. Hardness is carbonate hardness and no noncarbonate hardness is present. common cations and their associated anions are shown in table 22 2. theory the method described below relies on the competitive complexation of divalent metal ions by ethylenediaminetetraacetic acid (edta) or an indicator. the table 22 1. correlation of water hardness values.

Comments are closed.