Balancing Redox Reactions In Acid Redox Reactions And Electrochemistry

Balancing Redox Reactions In Acidic And Basic Conditions Youtube Solution. step 1: separate the half reactions. by searching for the reduction potential, one can find two separate reactions: cu (aq) e − → cu(s) and. fe3 (aq) 3e − → fe(s) the copper reaction has a higher potential and thus is being reduced. iron is being oxidized so the half reaction should be flipped. In order to get the electrons in each half reaction equal, one or both of the balanced half reactions will be multiplied by a factor. 2) duplicate items are always removed. these items are usually the electrons, water and hydrogen ion. after example #5c, i have a suggestion for searching for help on balancing a specific equation.

Electrochemistry 7 Balancing Redox Reactions In Acidic Medium Youtube This lecture examines how to balance complex redox reactions in both acidic and basic conditions. we examine everything from half reactions to the full balan. Balancing redox reactions, cont’d at this point, both ½ reactions should be balanced. the next step is to combine the two ½ reactions to form an overall equation. 6) multiply through each ½ reactions by appropriate coefficients to match e’s in each ½ reaction. 7) add ½ reactions and cancel e’s and other common species on left and right. Step 1a: write out the (unbalanced) reaction and identify the elements that are undergoing redox. step 1b: separate the reaction into two half reactions, balancing the element undergoing redox in each. step 2a: balance the oxygen atoms by adding water to one side of each half reaction. step 2b: balance the hydrogen atoms by adding h ions. Steps to balance: step 1: separate the half reactions that undergo oxidation and reduction. oxidation: i − i 2. this is the oxidation half because the oxidation state changes from 1 on the left side to 0 on the right side. this indicates a gain in electrons. reduction: mno − 4 mn2 .
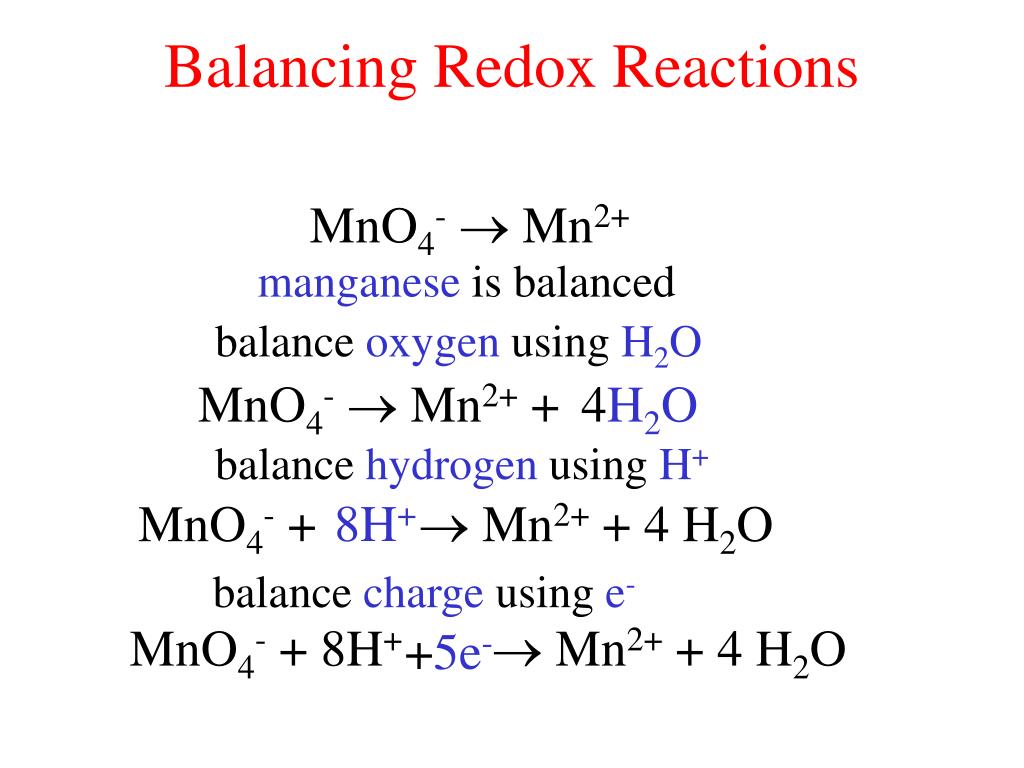
Ppt Balancing Redox Reactions Powerpoint Presentation Free Download Id 3199250 Step 1a: write out the (unbalanced) reaction and identify the elements that are undergoing redox. step 1b: separate the reaction into two half reactions, balancing the element undergoing redox in each. step 2a: balance the oxygen atoms by adding water to one side of each half reaction. step 2b: balance the hydrogen atoms by adding h ions. Steps to balance: step 1: separate the half reactions that undergo oxidation and reduction. oxidation: i − i 2. this is the oxidation half because the oxidation state changes from 1 on the left side to 0 on the right side. this indicates a gain in electrons. reduction: mno − 4 mn2 . Guidelines for balancing redox equations. step 1. write down the unbalanced equation. step 2. separate the redox reaction into half reactions. a) assign oxidation numbers for each atom. b) identify and write out all redox couples in reaction. c) combine these redox couples into two half reactions. step 3. This chemistry video tutorial shows you how to balance redox reactions under acidic conditions. it also shows you how to identify which half reaction is oxi.

Balancing Redox Reactions In Acidic And Basic Solution Youtube Guidelines for balancing redox equations. step 1. write down the unbalanced equation. step 2. separate the redox reaction into half reactions. a) assign oxidation numbers for each atom. b) identify and write out all redox couples in reaction. c) combine these redox couples into two half reactions. step 3. This chemistry video tutorial shows you how to balance redox reactions under acidic conditions. it also shows you how to identify which half reaction is oxi.

Comments are closed.