11 2 Standard Reduction Potential Chemistry Libretexts
11 2 Standard Reduction Potential Chemistry Libretexts Glossary standard cell potential \( (e^\circ \ce{cell})\) the cell potential when all reactants and products are in their standard states (1 bar or 1 atm or gases; 1 m for solutes), usually at 298.15 k; can be calculated by subtracting the standard reduction potential for the half reaction at the anode from the standard reduction potential for the half reaction occurring at the cathode. It is written in the form of a reduction half reaction. an example can be seen below where "a" is a generic element and c is the charge. standard reduction potential. ac ce− → a (1) (1) a c c e − → a. for example, copper's standard reduction potential of eo = 0.340 v) e o = 0.340 v) is for this reaction: cu2 2e− → cu (2.
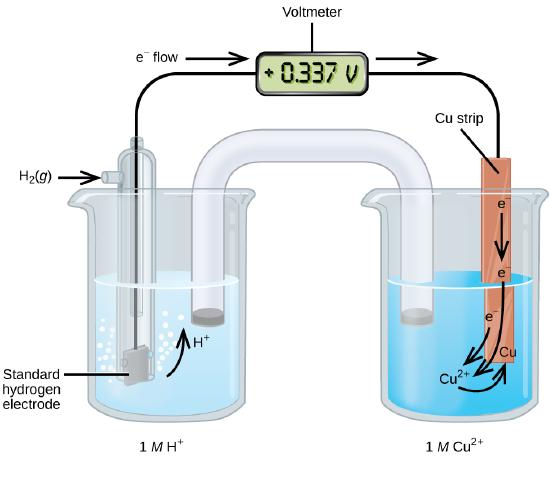
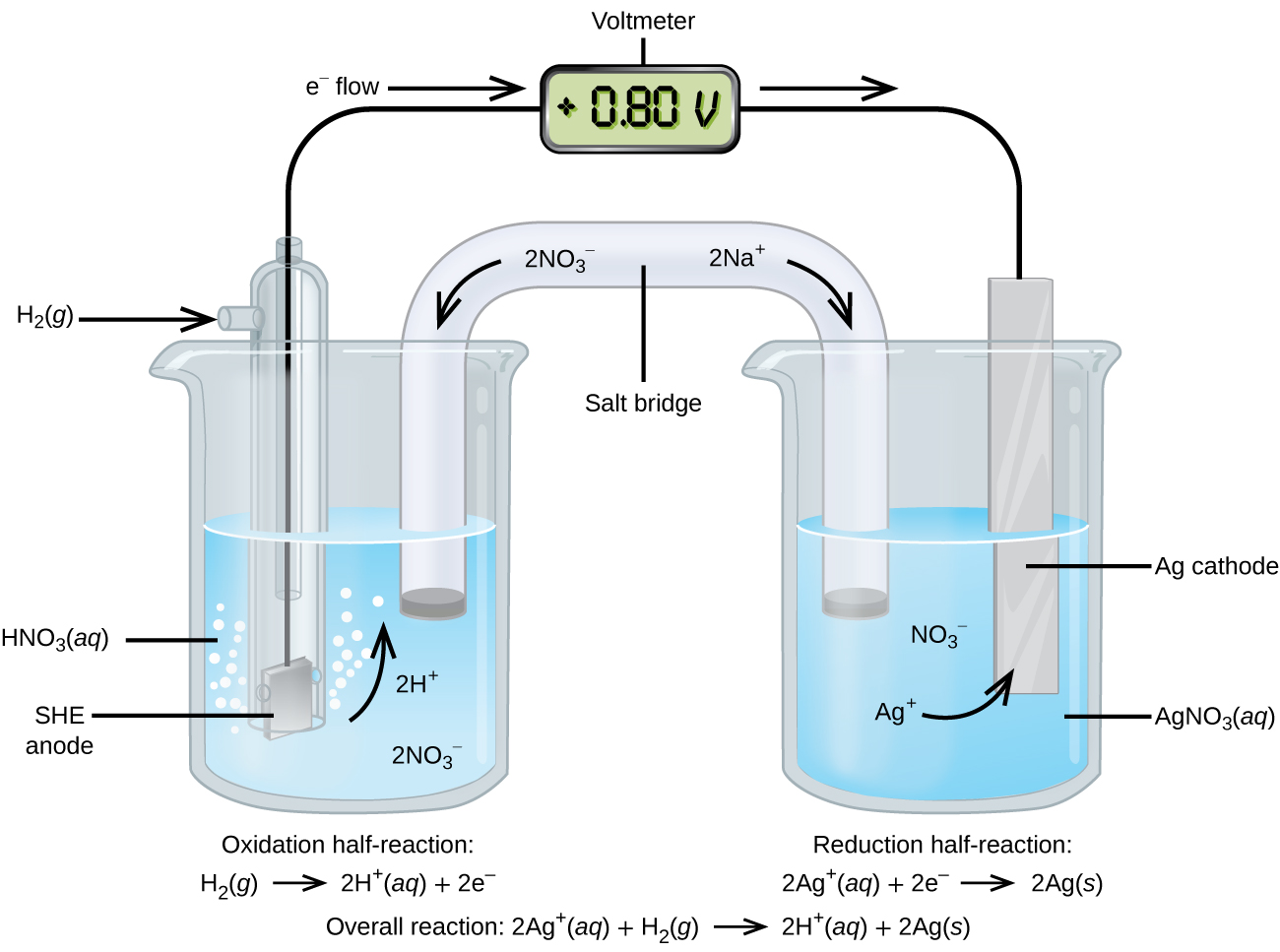
11 2 Standard Reduction Potential Chemistry Libretexts Contributors and attributions; the following table provides e o for selected reduction reactions. values are from the following sources: bard, a. j.; parsons, b. The she is rather dangerous and rarely used in the laboratory. its main significance is that it established the zero for standard reduction potentials. once determined, standard reduction potentials can be used to determine the standard cell potential, e cell °, e cell °, for any cell. for example, for the cell shown in figure 17.4,. Table 17.3.1 provides a listing of standard electrode potentials for a selection of half reactions in numerical order, and a more extensive listing is given in standard electrode (half cell) potentials. table 17.3.1. selected standard reduction potentials at 25 °c. half reaction. The standard reduction potentials for cu 2 and ag are 0.34 v and 0.80 v, respectively. calculate the cell potential at 25°c. calculate the cell potential at 25°c. solution:.

Comments are closed.