The Four Phases Of Clinical Trials Diversity In Clinical Trials Akf

The Four Phases Of Clinical Trials Diversity In Clinical Trials Akf Go It There are usually four phases of a clinical trial. each phase helps move the study along, step by step. the purpose of a clinical trial could be to study a m. Inclusion in clinical research is one way to address these health disparities. by intentionally including racial and ethnic minority groups, researchers may develop and refine more effective therapies. issues addressing the lack of diversity can be viewed from the standpoint of multiple stakeholders.
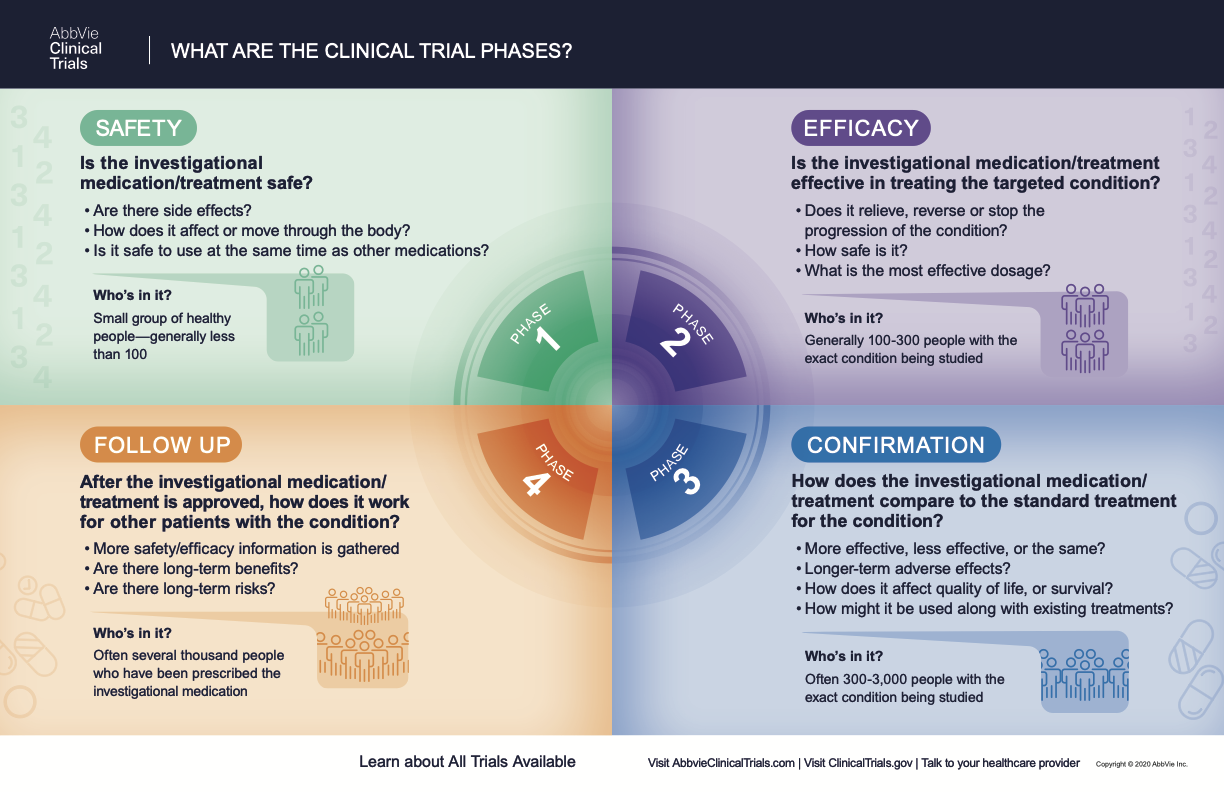
What Are The Phases Of Clinical Trials Clinical Trial Phases May 4, 2022. there are usually four phases of a clinical trial. each phase helps move the study along, step by step. the purpose of a clinical trial could be to study a medicine, a therapy, or a method of preventing or detecting a disease. before a clinical trial even starts, the new medicine or therapy is tested in a laboratory and with. Enhancing clinical trial diversity is a moral and scientific imperative. the lack of diversity is an obstacle to understanding the safety and efficacy of novel therapies across population subgroups, which is crucial to reducing disparities and advancing equity. furthermore, the striking and persistent under representation of minority racial and ethnic groups in clinical trials is harmful. in. What clinical trials are: clinical trials are research studies that test new treatments in people. before treatments can be approved to use with the public, they go through many phases of testing. what happens in clinical trials: during the trial, you will get care from a treatment team, including doctors, nurses and other health care providers. In june 2022, the u.s. house of representatives passed legislation intended to increase the diversity of the populations enrolled in clinical trials of new drugs. under this bill, study sponsors.

The Importance Of The 4 Phases Of Clinical Trials Learn About The Success Ra What clinical trials are: clinical trials are research studies that test new treatments in people. before treatments can be approved to use with the public, they go through many phases of testing. what happens in clinical trials: during the trial, you will get care from a treatment team, including doctors, nurses and other health care providers. In june 2022, the u.s. house of representatives passed legislation intended to increase the diversity of the populations enrolled in clinical trials of new drugs. under this bill, study sponsors. The purpose of a clinical trial could be to study a medicine, a therapy, or a method of preventing or detecting a disease. phase 1: small trial of 20 100 people why researchers observe the participants very closely, and check to see how the medicine or therapy works in a human body. phase 2: larger trial of about 300 people to gather more. Conclusions. in summary, the cdr framework provides an objective and transparent approach to evaluating clinical trial diversity. the use of this framework will elevate the conver sation on clinical trial diversity and enhance transparency and accountability, consequently promoting equity in trials of new drugs.
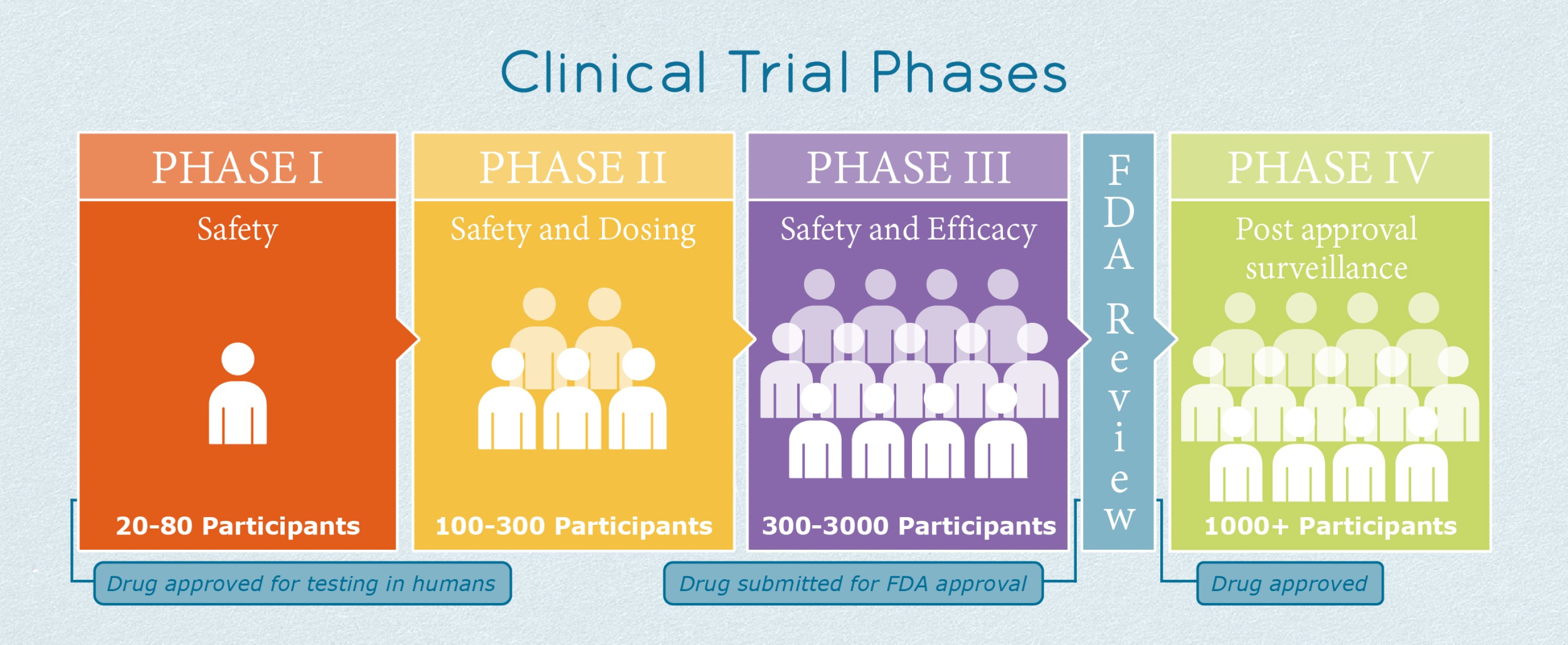
Phase 4 Clinical Trial The purpose of a clinical trial could be to study a medicine, a therapy, or a method of preventing or detecting a disease. phase 1: small trial of 20 100 people why researchers observe the participants very closely, and check to see how the medicine or therapy works in a human body. phase 2: larger trial of about 300 people to gather more. Conclusions. in summary, the cdr framework provides an objective and transparent approach to evaluating clinical trial diversity. the use of this framework will elevate the conver sation on clinical trial diversity and enhance transparency and accountability, consequently promoting equity in trials of new drugs.

Phases Of A Trial Treatment Lupus Clinical Trials

Comments are closed.