Solved 2 Answer The Questions Below For The Resonance Chegg

Solved 2 Answer The Questions Below For The Resonance Chegg See answer. question: 2. answer the questions below for the resonance structures shown below. no formal charges for any atom are shown in either resonance structure. a. determine the formal charge of each atom in each structure. (you do not have to show how you calculated this value.). Question: 2.9 fotmal changes in resonance structureschoose the resonance structure that results from the arrow pushing scheme below: . 2. 9 fotmal changes in resonance structures. choose the resonance structure that results from the arrow pushing scheme below: there are 2 steps to solve this one.

Solved The Answer To Each Question Below Praw Resonance Chegg Unlike o 3, though, the actual structure of co 32− is an average of three resonance structures. 1. because carbon is the least electronegative element, we place it in the central position: 2. carbon has 4 valence electrons, each oxygen has 6 valence electrons, and there are 2 more for the −2 charge. Exercise 2.14. draw the resonance contributors that correspond to the curved, two electron movement arrows in the resonance expressions below. exercise 2.15. in each resonance expression, draw curved two electron movement arrows on the left side contributor that shows how we get to the right side contributor. be sure to include formal charges. 3. below is the resonance for ch 3 coo , formal charges are displayed in red. the lewis structure with the most formal charges is not desirable, because we want the lewis structure with the least formal charge. 4. the resonance for hpo 3 2 , and the formal charges (in red). 5. the resonance for cho 2 1 , and the formal charges (in red). 6. Chemistry questions and answers. draw all the important resonance structures for the following ion showing all lone pairsof electrons, formal charges and double bonds. show the electron flow by using arrowsfor full credit. ( 6 points)fill in the boxes with the letter of the functional groups present in the following molecule: (2 points)a.

Solved Question 2 Please Answer The Following Questions Chegg 3. below is the resonance for ch 3 coo , formal charges are displayed in red. the lewis structure with the most formal charges is not desirable, because we want the lewis structure with the least formal charge. 4. the resonance for hpo 3 2 , and the formal charges (in red). 5. the resonance for cho 2 1 , and the formal charges (in red). 6. Chemistry questions and answers. draw all the important resonance structures for the following ion showing all lone pairsof electrons, formal charges and double bonds. show the electron flow by using arrowsfor full credit. ( 6 points)fill in the boxes with the letter of the functional groups present in the following molecule: (2 points)a. Our expert help has broken down your problem into an easy to learn solution you can count on. question: consider the net charge of the starting resonance structure below. the resulting resonance structure will have: a net positive charge no charge a net negative charge a partial positive charge. there are 2 steps to solve this one. 1 2 with a knowledge of k, t, and γ, as provided by eq.(1), the plot, and eq.(4), respectively, we can ω. 2. given Ω. 2, we find . 0. ω. 2 = k = Ω. 2 γ. 2. (5) 0. m then using eq.(2) to write Ω . in terms of the period and eq.(4) to write γin terms of the ”half life” of the oscillation, we find m= k ≈4.56 kg. (6) 4π. 2 t2.

Solved 2 Resonance Structures Are Important For Chegg Our expert help has broken down your problem into an easy to learn solution you can count on. question: consider the net charge of the starting resonance structure below. the resulting resonance structure will have: a net positive charge no charge a net negative charge a partial positive charge. there are 2 steps to solve this one. 1 2 with a knowledge of k, t, and γ, as provided by eq.(1), the plot, and eq.(4), respectively, we can ω. 2. given Ω. 2, we find . 0. ω. 2 = k = Ω. 2 γ. 2. (5) 0. m then using eq.(2) to write Ω . in terms of the period and eq.(4) to write γin terms of the ”half life” of the oscillation, we find m= k ≈4.56 kg. (6) 4π. 2 t2.
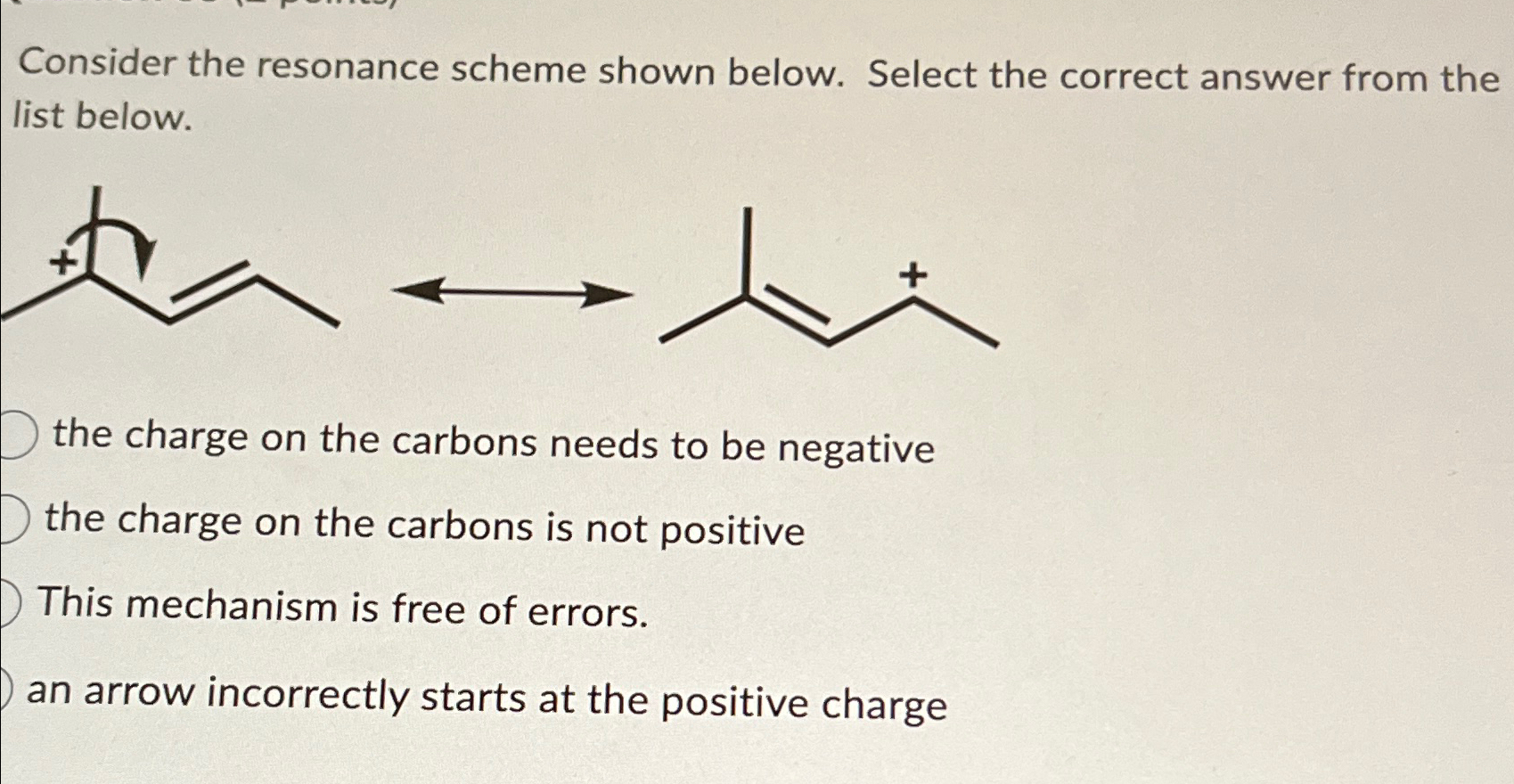
Solved Consider The Resonance Scheme Shown Below Select The Chegg

Comments are closed.