Phases Of Clinical Trials 30 Minutes E Course
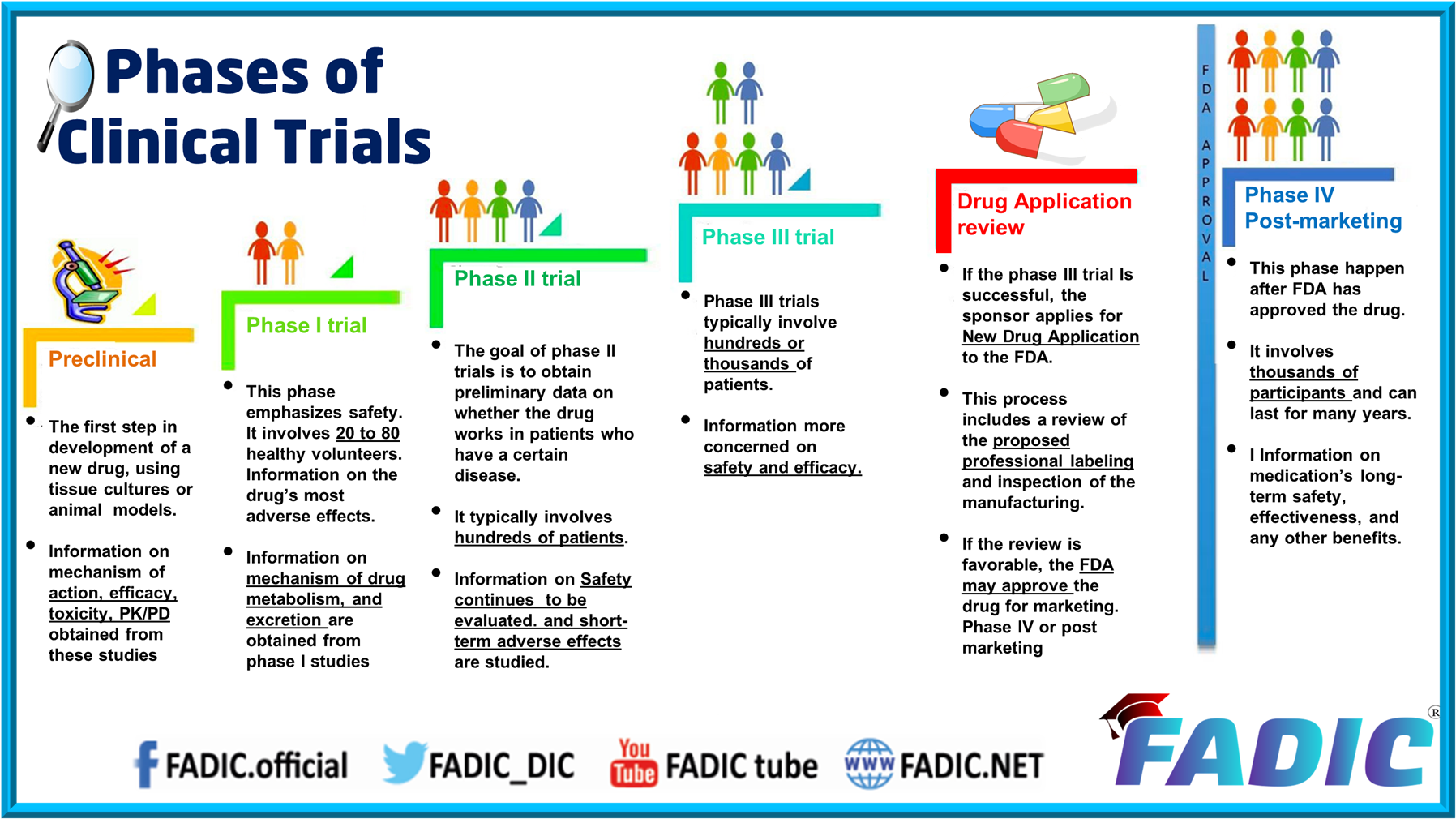
Phases Of Clinical Trials 30 Minutes E Course Phase 1. phase i trials were formerly referred to as “first in man studies” normally, a small group of 2–100 healthy volunteers will be recruited. these trials are often conducted in a clinical trial clinic, where the subject can be observed by full time staff. Begin your studies of clinical trials with an introduction to the background and rationale, a review of protocol development and trial registration, and an outline of a trial’s phases and stages. trial design. compare the advantages and disadvantages of different trial types and review three frameworks for clinical trials.

Phases Of Clinical Trials 30 Minutes E Course There are 6 modules in this course. clinical trials are experiments designed to evaluate new interventions to prevent or treat disease in humans. the interventions evaluated can be drugs, devices (e.g., hearing aid), surgeries, behavioral interventions (e.g., smoking cessation program), community health programs (e.g. cancer screening programs. There are 5 modules in this course. in this course, you’ll learn how to design and carry out clinical trials. each design choice has implications for the quality and validity of your results. this course provides you and your team with essential skills to evaluate options, make good design choices, and implement them within your trial. In summary, here are 10 of our most popular clinical trials courses. clinical trials operations: johns hopkins university. design and interpretation of clinical trials: johns hopkins university. clinical trials: good clinical practice: novartis. clinical trials analysis, monitoring, and presentation: johns hopkins university. Understanding the phases of clinical trials. in this guide, you will learn what clinical trials are, what types exist, and the details regarding the five different phases: phase 0, phase i, phase ii, phase iii, and phase iv. we address your frequently asked questions and explore related topics, and also include a clinical trial phase chart and.
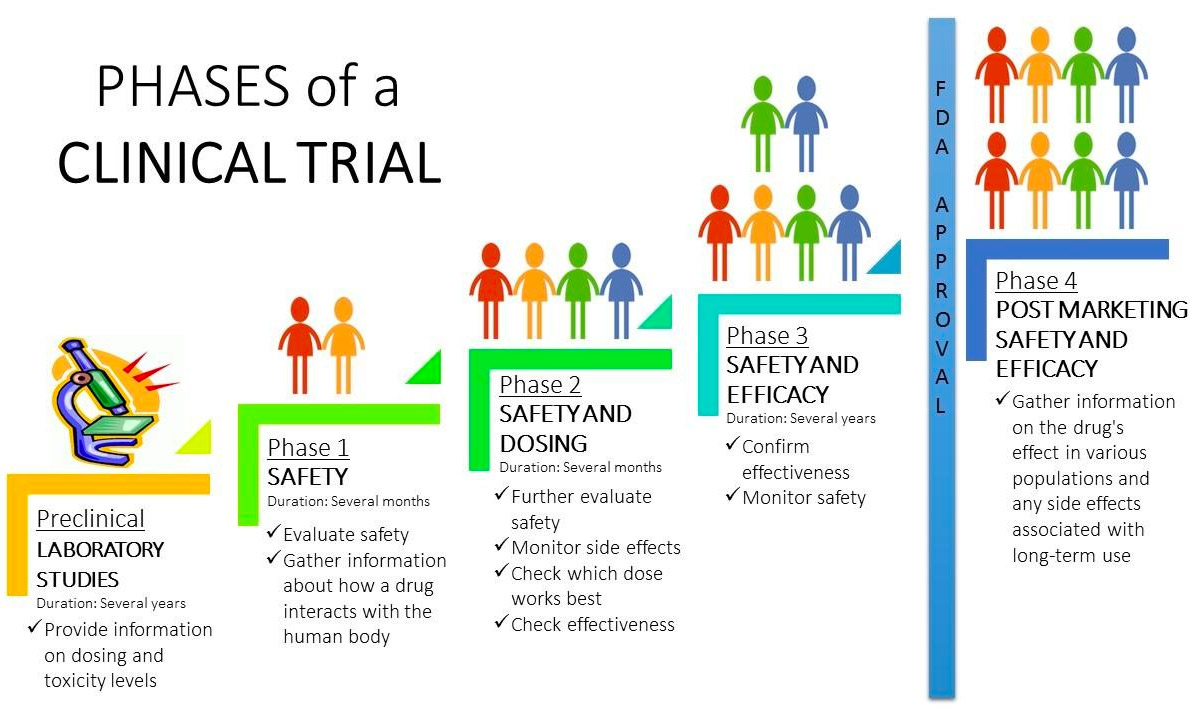
Clinical Trial Phases Diagram In summary, here are 10 of our most popular clinical trials courses. clinical trials operations: johns hopkins university. design and interpretation of clinical trials: johns hopkins university. clinical trials: good clinical practice: novartis. clinical trials analysis, monitoring, and presentation: johns hopkins university. Understanding the phases of clinical trials. in this guide, you will learn what clinical trials are, what types exist, and the details regarding the five different phases: phase 0, phase i, phase ii, phase iii, and phase iv. we address your frequently asked questions and explore related topics, and also include a clinical trial phase chart and. Clinical trials follow a particular timeline, from early, small scale, phase 1 studies to late stage, large scale, phase 3 studies.1 while there are many steps involved in the development of new drugs, clinical trials, which make up clinical research, are the part of drug development that involves people. here we describe the key goals and. Previously, he was a senior clinical program manager at gilead sciences where he oversaw management of all aspects of phase i iii global clinical trials and has led projects through several new drug applications (nda) and supplemental ndas. prior to that, he was a clinical research manager at abbott and a clinical trial manager at genentech.
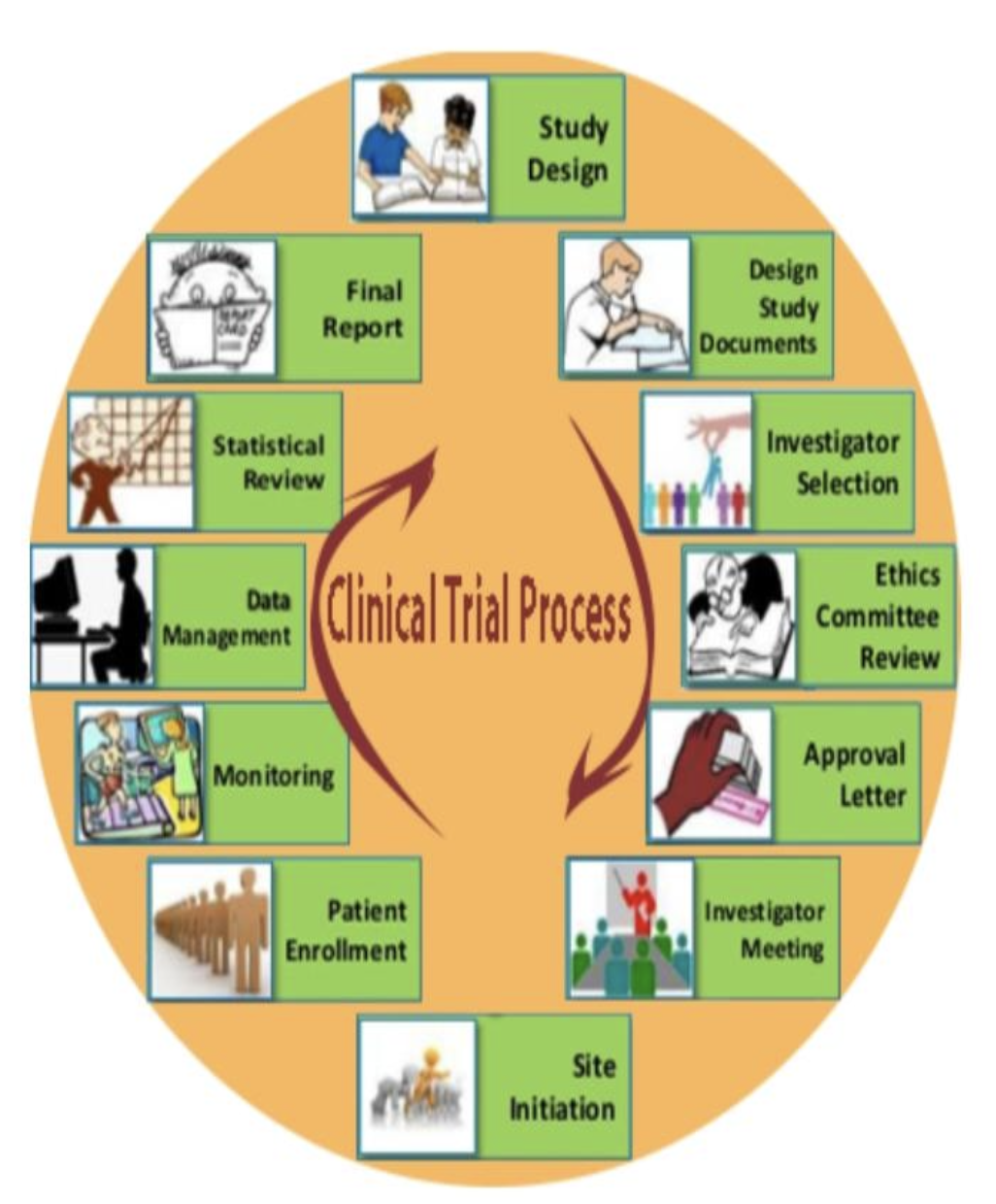
Fundamentals Of Clinical Trials Phases Of Clinical Trials Ccrps Clinical trials follow a particular timeline, from early, small scale, phase 1 studies to late stage, large scale, phase 3 studies.1 while there are many steps involved in the development of new drugs, clinical trials, which make up clinical research, are the part of drug development that involves people. here we describe the key goals and. Previously, he was a senior clinical program manager at gilead sciences where he oversaw management of all aspects of phase i iii global clinical trials and has led projects through several new drug applications (nda) and supplemental ndas. prior to that, he was a clinical research manager at abbott and a clinical trial manager at genentech.

Comments are closed.