Clinical Trial Phases Diagram
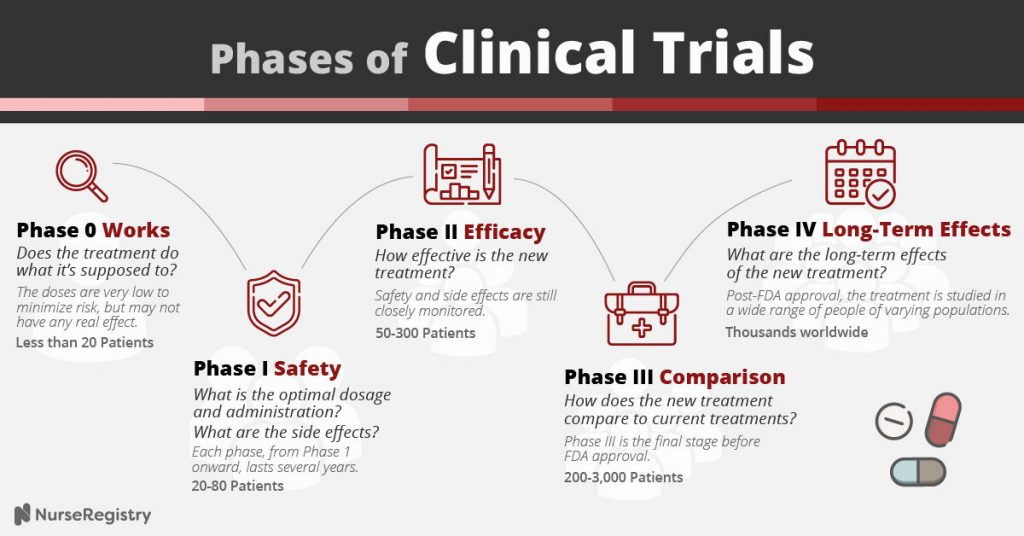
What Are The Different Phases Of A Clinical Trial Understanding the phases of clinical trials. in this guide, you will learn what clinical trials are, what types exist, and the details regarding the five different phases: phase 0, phase i, phase ii, phase iii, and phase iv. we address your frequently asked questions and explore related topics, and also include a clinical trial phase chart and. It consists of a first administration in a small group of human subjects with a certain disease or condition. phase 2a are the proof of concept studies and phase 2b are the definite dose finding studies. phase ii’s main goal is to test effectiveness (how well the drug works) and to further evaluate safety in a larger group of subjects.
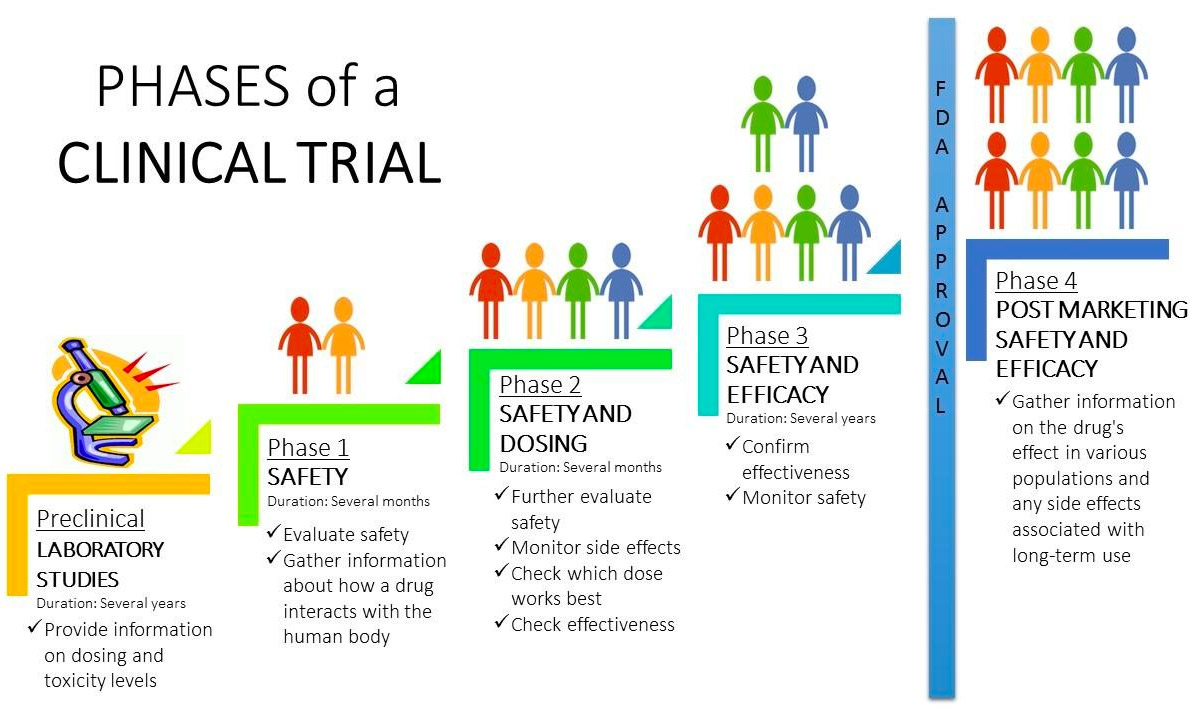
Clinical Trial Phases Diagram Phase 0 of a clinical trial is done with a very small number of people, usually fewer than 15. investigators use a very small dose of medication to make sure it isn’t harmful to humans before. Post approval trials that further investigate the therapeutic use of an investigational drug focus on: optimization of the drug monitoring of long term adverse events contraindicative drugs or diseases drug effectiveness and drug safety in of research further investigation of drug drug interactions, dose response and safety. Clinical trials follow a typical series from early, small scale, phase 1 studies to late stage, large scale, phase 3 studies. what are the clinical trial phases? watch this video to learn about. The process of learning about and developing an investigational medicine is divided into four phases. at first, very few people receive the medicine being studied. the number of people participating in clinical studies grows along with our understanding of the investigational medicine, and the research continues as long as the potential.

Phases Of A Trial Treatment Lupus Clinical Trials Clinical trials follow a typical series from early, small scale, phase 1 studies to late stage, large scale, phase 3 studies. what are the clinical trial phases? watch this video to learn about. The process of learning about and developing an investigational medicine is divided into four phases. at first, very few people receive the medicine being studied. the number of people participating in clinical studies grows along with our understanding of the investigational medicine, and the research continues as long as the potential. Clinical trials are usually conducted in phases that build on one another. each phase is designed to answer certain questions. knowing the phase of the clinical trial is important because it can give you some idea about how much is known about the treatment being studied. there are benefits and risks to taking part in each phase of a clinical. The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. [1] for drug development, the clinical phases start with testing for drug safety in a few human subjects, then expand to many study.
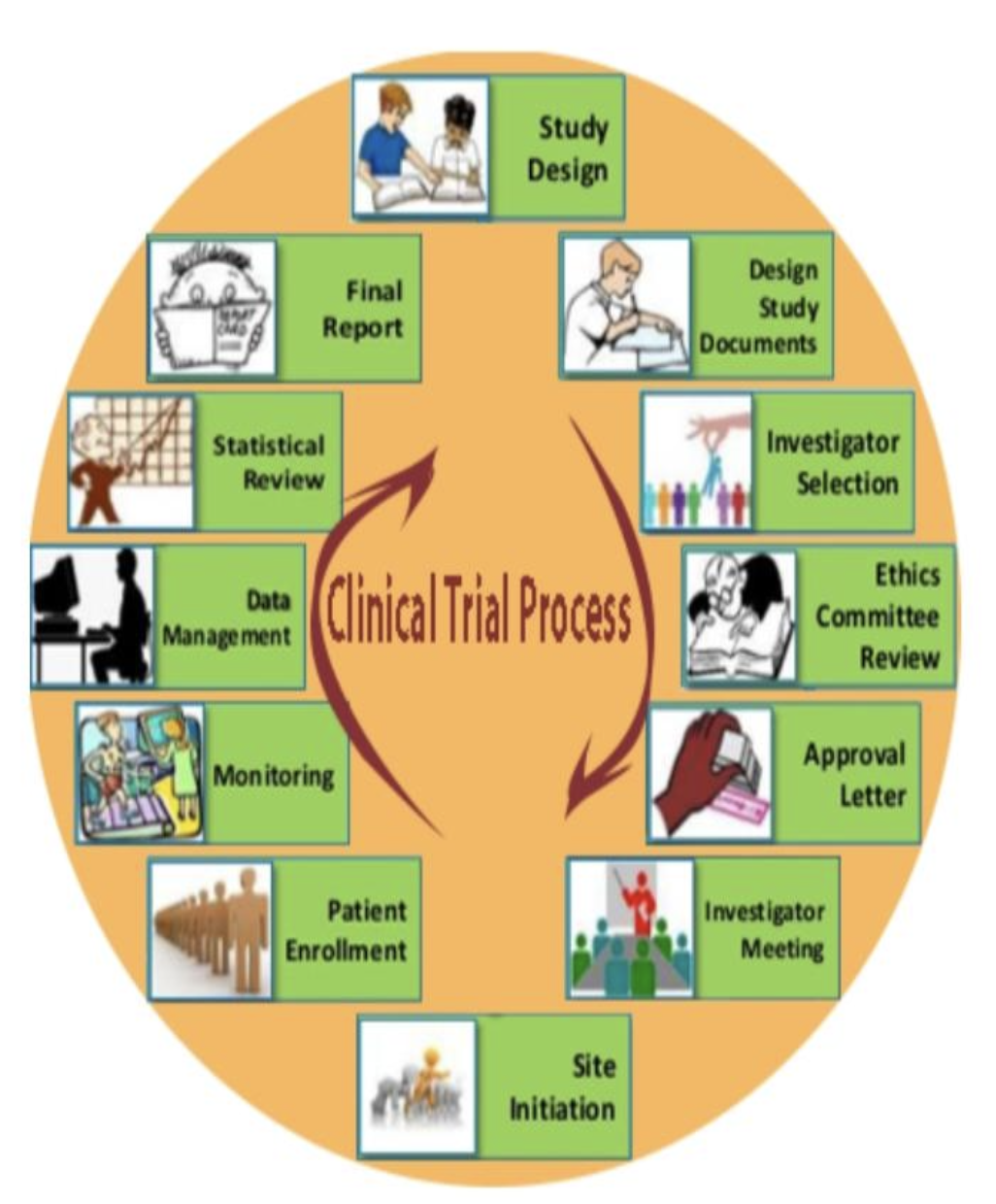
Fundamentals Of Clinical Trials Phases Of Clinical Trials Ccrps Clinical trials are usually conducted in phases that build on one another. each phase is designed to answer certain questions. knowing the phase of the clinical trial is important because it can give you some idea about how much is known about the treatment being studied. there are benefits and risks to taking part in each phase of a clinical. The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. [1] for drug development, the clinical phases start with testing for drug safety in a few human subjects, then expand to many study.

Comments are closed.